The Glad and Sad Parts of Collecting - Part III
by Joseph A. A. Borque, Sr.
Issue 299 - March 1998
Dear Reader,
In my last writing, Part II, of this article I mentioned that Cambridge Cobalt-1 fluoresced under Ultraviolet (UV) light, and contained a low radiation level Geiger counter reading while in the Xl ratio gauge setting (average: 0.08 reading). In order to show the contrast between this low Cobalt-I reading and a higher reading, I chose to measure the radioactive emission emanating from an item most probably containing URANIUM.
During the 1920s and 1930s, major glass manufacturers used COBALT OXIDE to make transparent blue glass of all shades, URANlUM OXIDE for transparent yellow (canary) and green transparent glass and URANIUM for translucent (near opaque) ivory glass. Glass made with these radioactive elements or compounds then became radioactive glass. They will fluoresce under a UV light, and will show a positive radioactive emission level on a Geiger counter meter and the bombardment of the Geiger counter tube can be heard through an earphone attached to the Geiger counter.
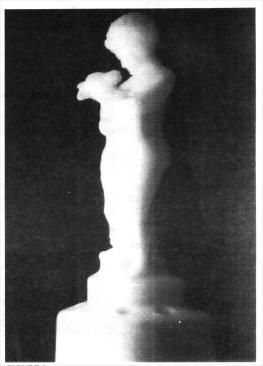
In order to show the contrast between these pieces of Cambridge Cobalt-1 with a low radioactive reading and a piece of Cambridge glass with a higher density reading, I chose an Ivory Two-kid flower holder, which gives off a strong radioactive reading of 0.55 to a 1.0 reading - (I had to step-up to the X40 ratio gauge reading to enable me to get a reading, as the bombardment of the radioactive rays overpowered the Xl gauge). This reading indicates that the Ivory Two-Kid holder emits about 11+ times more radioactive rays than those from the Cobalt-1 three-piece table-center Set.
Radioactive elements or compounds are measured by the half-life system. Radioactivity is the spontaneous disintegration or decay of the nucleus of an atom by emission of particles, usually accompanied by electromagnetic radiation. Natural radioactivity is exhibited by several elements to include URANIUM. The half-life I just mentioned is the time required for one half of the given radioactive substance to decay. In This example we are speaking of URANIUM which was most probably used to attain the ivory color of the Cambridge Two-Kid flower holder. URANIUM is a feebly active substance in contrast to RADIUM which is one of the most radioactive and poisonous substances known; yet both are related.
The half-life of RADIUM is about 1,690 years. The ultimate parent of RADIUM is the heavy element URANIUM, which it is always found to be associated with. (Encyclopedia Americana, 23-125-'57)
And now, Dear Reader, pay close attention to what I am about to relate. This Ivory Two-Kid flower holder, made by the Cambridge Oiass Company in circa 1930, was radioactive when it was manufactured about 66 years ago. Every moment of its existence to the very moment I write this article (January 1998), it has been continually emitting radioactive alpha and beta rays without ever stopping. Imagine - it has been doing this for 68 years as of this writing.
You may want to ask why is it doing this, and how long will it continue to do this. First Question: Why is it doing this? ... Here is why: About 58 years ago the batch mixer at the Cambridge Glass Company placed 2-1/2 pounds of a strong radioactive element, most probably URANIUM, into a glass-making batch weighing 4,237.25 pounds. (These figures as relate to weight may not be the exact ratio used by Cambridge. but good chances are they were close as these are the exact figures used by two well-known manufacturers of colored glass for their ivory glass formulas which were made during the same period - 1920s to mid-1940s. Most glass companies used the same or near same formulas for glass coloration, as these formulas were obtained for the asking from the element and chemical supply houses who worked very close to the glass manufacturers.)
All ingredients were carefully mixed together. They were melted down. A gaffer or gatherer scooped up a blob of the melt and placed it in a Two-Kid mold. A mold plunger was used to push the molten blob into every space within the mold. The mold was opened and, lo and behold, there stood the product of the very mold we are discussing, the Ivory Two-Kid flower holder!
 Even though the ratio of the radioactive material would only be
about 1 part to 494.0 parts of batch, the Two-Kid statue would become
saturated with the redioactive element. According to physicists, RADIUM
is the offspring of URANIUM. RADIUM emits alpha, beta, and gamma rays.
URANIUM emits alpha and beta rays.
Even though the ratio of the radioactive material would only be
about 1 part to 494.0 parts of batch, the Two-Kid statue would become
saturated with the redioactive element. According to physicists, RADIUM
is the offspring of URANIUM. RADIUM emits alpha, beta, and gamma rays.
URANIUM emits alpha and beta rays.
I conducted the following test to denote whether or not this Two-Kid figurine was emitting any beta rays. Alpha rays are readily absorbed in regular air space very quickly, and one thin sheet of paper will stop alpha rays. Some of the beta rays move with a velocity of over 170,000 miles per second, I took 65 pages (page 1 to 130) from my dictionary and found that the radioactive waves emitting from The Two-Kid statue went right through them, giving a good reading on the Geiger counter. One more test had to be accomplished, I took en empty 28-ounce heavy-duty metal can and placed it over the Two-Kid's upper body. The results: The radioactive rays went directly through the heavy-duty metal can, and I got a good reading on the other side on the Geiger counter. These were beta rays, with little doubt. The color of the Two-Kid statue is Ivory, the radioactive emission is strong and continuous, it fluoresces brightly under UV light. It allows a photograph to be taken of it in complete darkness at a speed of 1/8th of a second at f.8 with ISO 400 with nothing else but one overhead UV light. These facts enable me to opine that the radioactive element in this piece of Cambridge glass is most probably URANIUM.
It is quite shocking to believe that the half-life of RADIUM is 1,690 years, which means that it it is not destroyed through any other means, it should remain radioactive for about nine more half-lives or about 16,900 years.
It is known~that the ultimate parent of RADIUM is URANIUM. Bearing this in mind and knowing that the offspring of URANIUM is RADIUM Wilh half-life of 1,690 years, then what is the half-life of our beautiful Cambridge glass Two-Kid Ivory figure containing URANIUM? - Prepare yourself for a shocking answer: Several thousand million years before it is only half transformed. Just stop and think, this Cambridge glass flower holder in IVORY and ALL OTHER IVORY PIECES OF CAMBRIDGE owned by you and for any other members of National Cambridge Collectors will remain alive for EVERMORE!
The next and final writing of this article will deal with the "Sad Part of Collecting Glass". Until then, I hope you will be successful in "the hunt for Triangle-C glass."
'Til next time,
Joe
